Subproject 7
Micro-electrochemical investigation of O2 and CO2 reduction
Electrochemical reactions with high turnover can impose tremendous changes to the local reaction environment of catalytically active sites. These alterations become more severe with increasing reaction rate, e.g. in electrolyzers operated at high current densities. Such high-rate conversions are a key feature of gas-diffusion electrodes (GDEs) where gaseous reactants such as oxygen or carbon dioxide are vapor-fed into an electrolyte wetted pore structure. In these systems alterations in the reaction micro enironment manifest in increased hydroxide activities next to the electrode surface which are accompanied by lower water activities. These local dynamic changes as compared with the bulk electrolyte may affect not only reaction rates but also the selectivity of an electrocatalytic reaction. This is especially intriguing for the proton consuming carbon dioxide reduction reaction (CO2RR) as it can form a variety of different products, whereas the selectivity strongly depends on the pH value. Steering the CO2RR towards desired products thus requires understanding on how the environment of the catalytically active sites differs from the bulk conditions within the electrolysis cell.
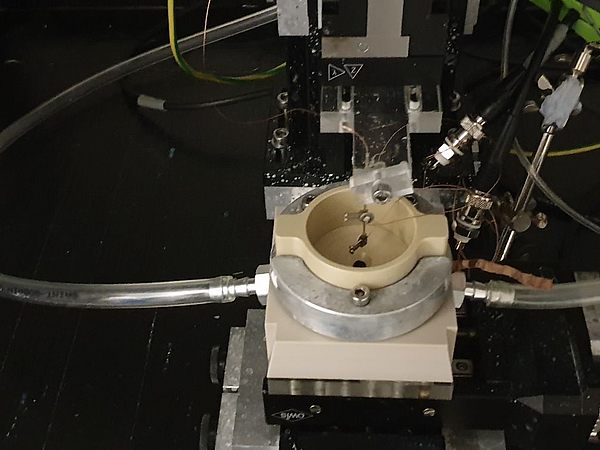
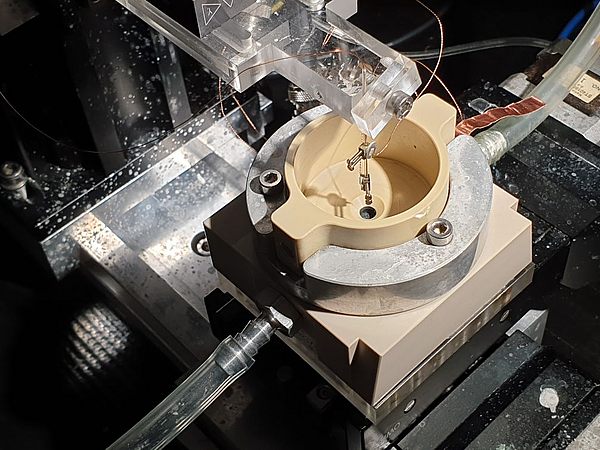
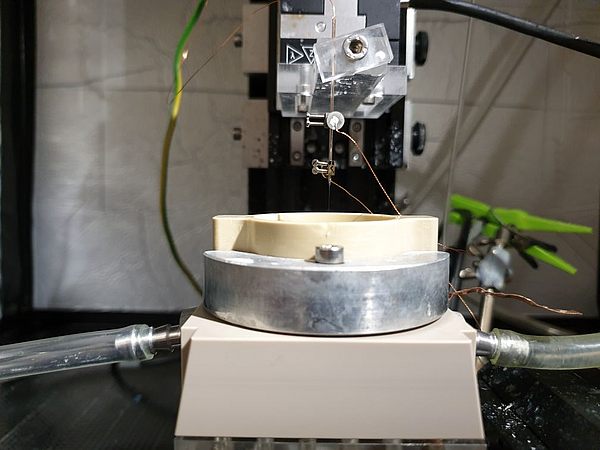
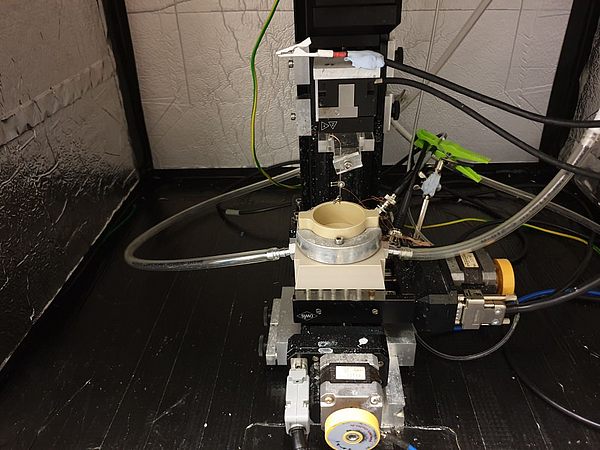
In general, the determination of pH-changes in close proximity to the electrode remains a challenging task, especially when considering dynamic interfaces as present in operating GDEs. During the first funding period, we introduced a scanning electrochemical microscopy (SECM) based technique employing Pt-microelectrodes as local probes to determine water and hydroxide activities above working Ag‑based GDEs during the oxygen reduction reaction (ORR). The electrochemical potential for Pt/Pt-oxidation and reduction at the Pt-tip electrode which is positioned in close proximity to the working GDE is monitored by cyclic voltammetric measurements and serves as a descriptor for the local pH conditions in its immediate sensing environment. [1]
During the current project phase, we adapt the previously developed concept for studying local reaction conditions to the CO2RR at Ag/PTFE based GDEs. Ag is known to yield a product mixture mainly composed of CO and H2, the main components of commercially available syngas. Two main scientific question arise: Firstly, how are local reaction species and the local pH value connected to the selectivity between both competing reactions? And secondly, can we develop and validate strategies to modulate the local reaction environment in order to deliberately steer CO/H2 product ratios? For this purpose, we utilize a SECM cell enabling the approach of Pt-tips to Ag-GDEs under CO2RR conversion conditions in CO2 gas breathing mode, while simultaneously enabling quantitative product analysis of the gas phase. Further, a conceptually new operando Raman cell is envisaged to acquire insights by vibrational spectroscopy studies of reaction intermediates and products. Based on the analytical access to the local reaction environment of CO2RR-GDEs, we will explore strategies for local pH modulation by controlled introduction of pH-influencing parasitic side reactions.
[1] A. Botz, J. Clausmeyer, D. Öhl, T. Tarnev, D. Franzen, T. Turek, W. Schuhmann, Angew. Chem. Int. Ed.2018, 57, 12285–12289.
Ruhr Universität Bochum
Lehrstuhl für Analytische Chemie – Center for Electrochemical Sciences
Universitätsstraße 150
Building NC 04/788
44780 Bochum
Fax: +49 234 32-14683
https://www.ruhr-uni-bochum.de/elan/#
Contact
Prof. Dr. Wolfgang Schuhmann
Phone: +49 234 32-26200
E-Mail: wolfgang.schuhmann@rub.de
Dr. Denis Öhl
Phone: +49 234 32-24151
E-Mail: Denis.Oehl@ruhr-uni-bochum.de
Stefan Dieckhöfer, M.Sc.
Phone: +49 234 32-24151
E-Mail: Stefan.Dieckhöfer@ruhr-uni-bochum.de